Shockwave therapy and hip arthritis
posted: May 06, 2022.
Extracorporeal shockwave therapy shows regeneration in hip necrosis
C.-J. Wang, F.-S. Wang, J.-Y. Ko, H.-Y. Huang, C.-J. Chen, Y.-C. Sun, Y.-J. Yang
Rheumatology, Volume 47, Issue 4, April 2008, Pages 542–546, https://doi.org/10.1093/rheumatology/ken020
Published:
Abstract
Objectives. The effect of shockwave in osteonecrosis of the femoral head (ONFH) is poorly understood. The purpose of this study was to investigate the regeneration effects of shockwave in ONFH.
Methods. This study consisted of 14 femoral heads from 14 patients undergoing total hip arthroplasty for ONFH. Seven patients with seven hips who received shockwave prior to surgery were designated as the study group, whereas, seven patients with seven hips who did not receive shockwave were assigned to the control group. Both groups showed similar demographic characteristics. The femoral heads were investigated with histopathological examination and immunohistochemical analysis with von Willebrand factor (vWF), VEGF, platelet endothelial cell adhesion molecule-1 (PECAM-1) also referred to as (CD 31) and vascular cell adhesion molecule (VCAM) for angiogenesis, and with proliferation cell nuclear antigen (PCNA), Dickkopf-1 (DKK1) and Winless 3a (Wnt 3) for bone remodelling and regeneration.
Results. In histopathological examination, the study group showed significantly more viable bone and less necrotic bone, higher cell concentration and more cell activities including phagocytosis than the control group. In immunohistochemical analysis, the study group showed significant increases in vWF (P < 0.01), VEGF (P = 0.0012) and CD 31 (P = 0.0023), Wnt3 (P = 0.008) and PCNA (P = 0.0011), and decreases in VCAM (P = 0.0013) and DKK1 (P = 0.0007) than the control group.
Conclusions. Shockwave treatment significantly promotes angiogenesis and bone remodelling than the control. It appears that application of shockwave results in regeneration effects in hips with ONFH.
Extracorporeal shockwave, Regeneration, Osteonecrosis, Femoral headTopic:- angiogenesis
- vascular endothelial growth factor a
- von willebrand factor
- bone remodeling
- antigens
- cd31 antigens
- demography
- femoral head
- femur head necrosis
- hip region
- hip joint
- necrosis
- phagocytosis
- proliferating cell nuclear antigen
- surgical procedures, operative
- vascular cell adhesion molecule-1
- hip replacement arthroplasty
- extracorporeal shockwave therapy
- shock wave
- histopathology tests
Introduction
Treatment of osteonecrosis of the femoral head (ONFH) remains controversial [1]. Conservative treatments are generally unsuccessful, and surgery is indicated in symptomatic hips with the type of procedure varying according to the stage of the disease on image studies [2–4]. For early ONFH, femoral head-preserving procedures including core decompression, vascularized or non-vascularized bone graft and osteotomy are recommended [1–4]. The results of femoral head-preserving procedures varied considerably, and most studies reported less satisfactory outcomes [5–13]. For late cases, total hip arthroplasty (THA) is usually performed [14]. In young active patients, the complications of THA are common including thigh pain, polyethylene wear, osteolysis and component loosening [15]. Therefore, an effective and non-invasive method of treatment appears very attractive.
Extracorporeal shockwave therapy (ESWT) was shown to be more effective than core decompression and non-vascularized bone grafting for early ONFH [16]. We hypothesized that ESWT may result in regeneration of the femoral head with the improvement in blood supply. The purpose of this study was to investigate the regeneration effect of shockwave in hips with ONFH.
Materials and methods
The Ethical Committee of the Institutional Review Board on Human Studies of our hospital approved this study and written informed consent was acquired from all subjects according to the Declaration of Helsinki.
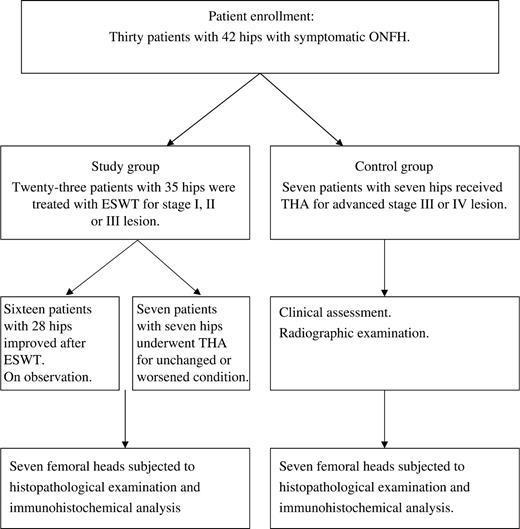
Between July 2004 and June 2005, 30 patients with 42 hips were treated for symptomatic ONFH at our hospital. Twenty-three patients with 35 hips with stage I, II or III lesion were treated with ESWT. The source of shockwave was from an OssaTron orthotripter (Sanuwave, Alpharetta, GA, USA). The treatment was performed on the operation table under general anaesthesia. The hip joint was properly positioned by adduction and internal or external rotation of the affected leg. The femoral artery was identified with digital palpation and confirmed with ultrasound Doppler, and was protected from direct shockwave contact. The junctional zone between avascular and normal bones of the femoral head was delineated with C-arm imaging. Four points with 1.0 cm apart within the zone were chosen with a metallic pin under C-arm imaging, and the corresponding locations were marked on the skin in the groin area. The depth of treatment was determined by adjusting the height of the table until the two ring markers of the device synchronized under C-arm imaging. Surgical lubricant was applied to the skin in contact with the shockwave tube. Each of the four locations was treated with 1500 impulses of shockwave at 28 kV (equivalent to 0.62 mJ/mm2 energy flux density), and a total of 6000 shocks were applied to the femoral head as a single session [16]. After treatment, patients walked with partial weight bearing on the affected leg for 4–6 weeks. Non-narcotic analgesic such as acetaminophen were prescribed for pain. The results showed improvement in 16 patients with 28 hips and un-improved or worsened in seven patients with seven hips. There was no device-related problem. There was no systemic or neurovascular complication. Local complications included ecchymosis in five and local swelling in six, and all spontaneously resolved within a few days.
THA was performed in seven patients with seven hips of the ESWT-treated group due to failure of treatment. The time interval from ESWT to THA ranged from 12 to 24 months. In addition, seven patients with seven hips with advanced stage III or IV lesion were initially treated conservatively with analgesics and protected from weight bearing to the affected leg, and THA was performed when the symptoms became unbearable. The time interval from the initial visit to THA ranged from 4 to 20 months. The patient selection flow diagram is shown in Fig. 1. This study consisted of 14 femoral heads from 14 consecutive patients with 14 hips undergoing THA for symptomatic ONFH. Among them, seven patients with seven hips who received ESWT prior to THA were assigned to the study group, whereas the other seven patients with seven hips who did not receive ESWT prior to THA were assigned to the control group. Both groups showed similar demographic characteristics as shown in Table 1.
Patient demographic characteristics
| Study group | Control group | P-value | |
|---|---|---|---|
| Number of hips | 7 | 7 | 1.00 |
| Sex (M/F) | 6/1 | 5/2 | 0.515 |
| Age (yrs) | |||
| Mean ± S.D. (range) | 41.3 ± 11.4 (19–56) | 39.9 ± 11.8 (21–57) | 0.411 |
| Body weight (kg) | |||
| Mean ± S.D. (range) | 67.7 ± 11.4 (59–80) | 65.1 ± 15.8 (42–89) | 0.36 |
| Body height (cm) | |||
| Mean ± S.D. (range) | 164.3 ± 6.1 (155–171) | 164.9 ± 7.8 (156–175) | 0.44 |
| Duration of disease (months) | |||
| Mean ± S.D. (range) | 18.7 ± 9.2 (12–28) | 16.3 ± 8.8 (8–30) | 0.278 |
| Stage of lesion | |||
| III | 5 | 2 | 0.063 |
| IV | 2 | 5 | 0.063 |
| Size of lesion (percentage of total surface) | |||
| Mean ± S.D. (range) | 42.0 ± 10.9 (27–53) | 48.4 ± 24.1 (20–76) | 0.269 |
| Aetiology | |||
| Steroid induced | 4 | 4 | 1.00 |
| Non-steroid | 3 | 3 | 1.00 |
M, male; F, female.
Open in new tabThe femoral heads were investigated with histopathological examination and immunohistochemical analysis for angiogenesis with von Willebrand factor (vWF), VEGF, platelet endothelial cell adhesion molecule-1 (PECAM-1) also referred to as (CD 31) and vascular cell adhesion molecule (VCAM) and for bone remodelling and regeneration with proliferation cell nuclear antigen (PCNA), Dickkopf-1 (DKK1) and Winless 3a (Wnt 3).
Histopathological examination
The bone specimens were decalcified and embedded in paraffin for section. The microsections were stained with haematoxylin–eosin (HE) stain. The histopathological features were examined by a bone pathologist blinded to the nature of the study. The microscopic features included tissue distributions of viable and necrotic bones, cartilaginous and fibrous tissues, cell concentration and cell activities including phagocytosis.
Immunohistochemical stain
The harvested specimens were fixed in 4% phosphate buffer solution (PBS)-buffered paraformaldehyde for 48 h and decalcified in PBS-buffered 10% ethylenediaminetetraacetic acid (EDTA). Decalcified tissues were embedded in paraffin. The specimens were cut longitudinally into 5-μm thick sections and transferred to polylysine-coated slides. Sections of the specimens were immunostained with specific reagents for vWF, VEGF, CD 31 and VCAM to identify angiogenesis and angiogenesis-related growth and proliferating indicators; and for PCNA, DKK1 and Wnt 3 to examine bone remodelling and regeneration (Santa Cruz Biotechnology Inc., CA, USA). The immunoreactivity in specimens was demonstrated using a horseradish peroxidase (HRP)-3′-,3′-diaminobenzidine (DAB) cell and tissue staining kit (R & D Systems, Inc., MN, USA). The immunoactivities were quantified from five areas in three sections of the same specimen using a Zeiss Axioskop 2 plus microscope (Carl Zeiss, Gottingen, Germany). All the images of each specimen were captured using a Cool CCD camera (SNAP-Pro c.f. Digital kit; Media Cybernetics, MD, USA). Images were analysed using an Image-Pro® Plus image-analysis software (Media Cybernetics). The percentage of positive immunolabelled cells over the total cells in each area was counted. Two pathologists blinded to the treatment regimens performed the measurements on all sections.
Statistical analysis
A power analysis revealed that a sample size of seven is adequate to establish the statistical significance with α = 0.05 and power = 0.8 with calculation based on the data provided in this study. The data between the hips with ESWT prior to THA and hips without ESWT are compared statistically using an independent t-test with the statistical significance at P < 0.05.
Results
The results of histopathological examination are summarized in Table 2. The ESWT group showed significantly more viable bones with live osteocytes and less necrotic bones with empty lacunae and apoptotic cells than the control group. Considerably higher cell concentration and more cell activities including phagocytosis were observed in ESWT group than the control group (Fig. 2).
Microscopic findings with HE stain showed significantly more viable bone and cell concentration and cell activity in study group than the control group.
The results of histopathological examination
| Shockwave (+) (n = 7) | Shockwave (−) (n = 7) | ||||
|---|---|---|---|---|---|
| Mean ± S.D. | 95% CI | Mean ± S.D. | 95% CI | P-value | |
| Viable bone (%) (range) | 45 ± 11.9 (30–60) | 42.8 ± 11.3 (29–57) | 23.3% ± 16.8 (0–60) | 22.1 ± 15.9 (0–57) | 0.014 |
| Necrotic bone (%) (range) | 17.1 ± 7.6 (10–30) | 16.3 ± 7.2 (9.5–29) | 41.1 ± 19.3 (20–70) | 39.1 ± 18.4 (19–67) | 0.0047 |
| Cartilage (%) (range) | 5.7 ± 10.2 (0–25) | 5.4 ± 9.7 (0–24) | 3.9 ± 4.9 (0–10) | 3.7 ± 4.6 (0–10) | 0.4206 |
| Fibrosis (%) (range) | 18.6 ± 7.5 (10–30) | 17.6 ± 7.1 (10–29) | 22.8 ± 9.4 (10–40) | 21.6 ± 8.9 (10–38) | 0.3523 |
| Phagocytic histiocyte (%) (range) | 13.6 ± 10.3 (5–35) | 12.9 ± 9.8 (5–33) | 7.5 ± 16 (5–15) | 6.3 ± 15.2 (4.8–14) | 0.2000 |
The results of immunohistochemical analysis are summarized in Table 3. The study group showed significant increases in vWF (P < 0.01), VEGF (P = 0.0012) and CD 31 (P = 0.0023), and a decrease in VCAM (P = 0.0013) than the control group. The results suggested that ESWT significantly promotes angiogenesis with new vessel formation and increases the angiogenesis-related growth factors. The study group also showed significant increases in PCNA (P = 0.0011) and Wnt 3 (P = 0.008) and a decrease in DKK1 (P = 0.0007) than the control. The results suggested that ESWT significantly promotes bone remodelling and regeneration. The microscopic features of the immunohistochemical stains for vWF, VEGF, CD 31, VCAM, PCNA, DKK1 and Wnt3 are shown in Figs 3–9, respectively.
Microscopic findings with vWF stain showed significantly more new vessels (angiogenesis) in the study group than the control group.
Microscopic features with immunohistochemical stain showed significantly higher VEGF expressions in the study group than the control group.
Microscopic features with immunohistochemical stain showed significantly more CD 31 expressions in the study group than the control group.
Microscopic features with immunohistochemical stain showed significantly less VCAM expressions in the study group than the control group.
 Open in new tabDownload slide
Open in new tabDownload slideMicroscopic features with immunohistochemical stain showed significantly more PCNA expressions and cell proliferations in the study group than the control group.
Microscopic features with immunohistochemical stain showed significantly less DKK1 expressions in the study group than the control group.
Microscopic features with immunohistochemical stain showed significantly more Wnt3 expressions in the study group than the control.
The results of immunohistochemical analysis
| Shockwave (+) (n = 7) | Shockwave (−) (n = 7) | ||||
|---|---|---|---|---|---|
| Mean ± SD | 95% CI | Mean ± SD | 95% CI | P-value | |
| VWF (%) (range) | 66 ± 5 (59–70) | 63 ± 4.7 (56–67) | 31 ± 5.3 (29–39) | 29 ± 5.1 (26–37) | <0.01 |
| VEGF (%) (range) | 87 ± 5.4 (79–94) | 83 ± 5.2 (75–89) | 64 ± 9.3 (49–72) | 60 ± 8.8 (47–68) | 0.0012 |
| CD 31 (%) (range) | 62 ± 20 (35–92) | 59 ± 19 (33–87) | 11 ± 4 (6–16) | 10.5 ± 3.6 (6–15) | 0.0023 |
| VCAM (%) (range) | 12 ± 5 (8–20) | 11.6 ± 4. 5 (8–19) | 29 ± 7 (20–38) | 27.9 ± 6.6 (19–36) | 0.0013 |
| PCNA (%) (range) | 85 ± 4.5 (78–90) | 80 ± 4.3 (74–86) | 62 ± 9 (52–73) | 59 ± 8.5 (49–69) | 0.0011 |
| DKK1 (%) (range) | 26 ± 11 (12–36) | 25 ± 10 (12–34) | 71 ± 11.8 (61–85) | 67 ± 11.2 (58–81) | 0.0007 |
| Wnt3 (%) (range) | 55 ± 1.1 (53–55) | 52 ± 1.0 (50–53) | 35 ± 8 (25–42) | 34 ± 7.6 (24–40) | 0.0080 |
The data are in mean ± S.D. (range).
